“Formamidinium lead iodide is a very good material for photovoltaic cells, but getting the correct and stable crystal structure is a challenge,” according to the University of Groningen.
“The techniques developed so far have produced rather poor results.”
The poor result is a mixture of two forms of the perovskite, one photo-active and the other not – depending on the relative positions of various ions within the crystal structure.
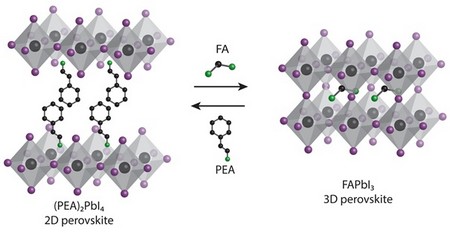
To increase the proportion of ‘good’ perovskite, researcher Sampson Adjokatse starts with a different perovskite, with a larger 2‑phenylethylammonium ion instead of formamidinium.
This forms perovskite with the right bits in the right places after it has been squeegeed onto a flat surface and heat annealed into a ~500nm film. “The important point is that these films are very smooth with large crystalline domains of up to 15μm,” said Adjokatse.
Formamidinium
Dipping into a formamidinium iodide solution causes formamidinium ions to replace 2‑phenylethylammonium.
“These films show much higher photoluminescence compared to reference FAPbI3 films and show increased stability when exposed to light or moisture,’ said fellow researcher Antonietta Loi. “This means that we now have a method for the production of high-quality films for perovskite solar cells using an industrially scalable technique.”
For more information, find yourself a copy of ‘Scalable fabrication of high-quality crystalline and stable FAPbI3 thin films by combining doctor-blade coating and the cation exchange reaction‘, published in the journal Nanoscale.
 Electronics Weekly Electronics Design & Components Tech News
Electronics Weekly Electronics Design & Components Tech News



