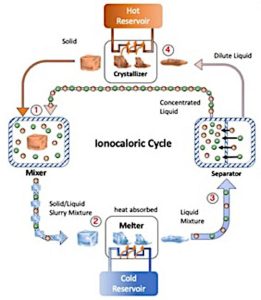
The technology has been dubbed ‘ionocaloric cooling’.
While warming and cooling a solid, liquid or gas can be used to shift some heat, much more can be shifted by a ‘phase change’ – for example: turning a liquid into a gas and back – as in a domestic fridge, or turning a solid into a liquid and back – for which the Berkeley Lab researchers have just presented their technique.
In the Berkeley case, ethylene carbonate has been chosen as the caloric material, and adding ions to it has been chosen as the way to melt it, which makes it colder – hence ‘ionocaloric’ (other research teams are studying ‘magnetocaloric’ and electrocaloric’ effects as alternative routes to this melting). Extracting the ions makes a warmer solid that can be used to dump any gathered heat.
The salt sodium iodide has been chosen as a source of ions.
A thermal effect needs a thermodynamic cycle if it is to do any useful cooling, and the team has designed a four-step Stirling cycle to use its ionocaloric effect, in which the working materials remain pumpable at all times.
“There is no carrier liquid for the elthylene carbonate sodium iodide combination, just the two materials,” project PhD student Drew Lilley told Electronics Weekly. “In general, the combination exists as a two phase mixture, like a slurry, so there is always some melted elthylene carbonate that acts as a carrier fluid.”
In the Berkeley four-step Stirling cycle:
- The salt is mixed with particles of the solid, which causes the temperature of the mixture to drop (think table salt and crushed ice) as Na+ and I- ions enter the solid particles
- This self-cooled slush is pumped to wherever heat must be removed, where the solid particles melt as they gather heat from their surroundings (the interior of a fridge, for example).
- The now-liquid is moved to where desalination is used to pull ions out of the elthylene carbonate, leaving two things: a warmed ion-rich liquid which is ready for a repeat of step one, and a separate stream of warmed liquid ethylene carbonate which is pumped on to step four where…
- …the liquid ethylene carbonate is passed though a heat exchanger, where heat is dumped, and it turns back into solid particles also ready for repeating step one
Apart from any pumping necessary for a practical refrigerator, it is the desalination process which needs energy.
In the Berkeley Lab case, an electrolytic cell desalinator has been designed, where a potential difference causes electron flow though the liquid.
Using a neat bit of electrochemistry, combined with a series of anion and cation exchange membranes (acquire the paper mentioned below for details) both sodium ions and iodine ions end up on one side of a membrane (ready to be pumped away for re-use), while the ethylene carbonate ends up concentrated in solution on the other side of the membrane (ready to solidify in the heat dumping step).
In practice, both streams leaving the desalinator are mixtures of ethylene carbonate, sodium iodide, and Na+, I– and I3- ions – with different balances in each stream.
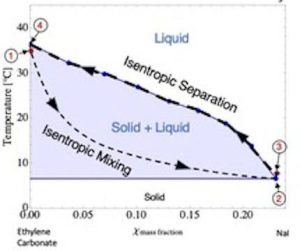
“We demonstrated a practical system using an ionocaloric Stirling refrigeration cycle. Our experimental results show a coefficient of performance of 30% relative to Carnot and a temperature lift as high as 25°C using a ~0.22V,” said the researchers in ‘Ionocaloric refrigeration cycle‘, a paper that describes the research and the underlying theory in the journal Science.
“We have this thermodynamic cycle and framework that brings together elements from different fields, and we’ve shown that it can work,” said Berkeley Lab researcher Ravi Prasher. “Now, it’s time for experimentation to test different combinations of materials and techniques to meet the engineering challenges.”
Prasher and Lilley have a provisional patent for ionocaloric refrigeration and have sketched out ways to use it in a practical refrigerator as well as identified criteria for selecting the active materials. “They calculated that it has the potential to compete with, or even exceed, the efficiency of gaseous refrigerants found in the majority of systems today,” according to the lab.
Diagrams provided by Drew Lilley, Lawrence Berkeley National Laboratory
…
 Electronics Weekly Electronics Design & Components Tech News
Electronics Weekly Electronics Design & Components Tech News


Slight problem – a fridge has a temperature lift of up to 50degC and a freezer even more. With only 25degC the heatsink on the back of the fridge would have to weigh more and need noisy fans to move the heat into the atmosphere.
Afternoon Mike
Indeed, and it requires sludge to be pumped, but my guess is that the first compressor-based refrigeration experiments were a little underwhelming.
My hope is that this is a little acorn 🙂
You can’t beat the laws of thermodynamics. Same as these air-source heat pumps the government wants everyone to have. We have one on the farm and guess what, it’s efficiency goes negative at -4 deg C. Fortunately it’s not the main heating system but from mid-spring to mid-autumn it makes sense when run from solar panels.
The only way the method proposed could work is with a 2 stage system which would double the volume and cost. Far better to focus on scaling down commercial CO2 based systems for home use as I gather there’s rather a lot of that gas going spare.
You made me think again Mike, and I have changed that headline too.
The lab emphasised that it had the potential to be used in fridges, and I did put a question mark after that claim in the headline, but I have removed it as it is very early days. (plus my knowledge of thermodynamics is… well, I will sum it up inside the following brackets: ( )