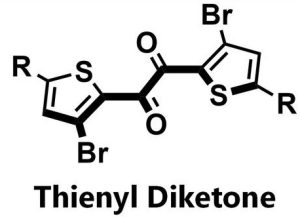
Its researchers came across a molecule, thienyl diketone, which is a champion among organic phosphorescers.
“We discovered this molecule by chance and initially did not understand why it demonstrated such superior performance,” said Osaka scientist Yosuke Tani. “However, as our research progressed, we began to connect the pieces.”
So far, only optically-induced emission has been shown, but the team points out that it looks promising for electrically-stimulated OLEDs.
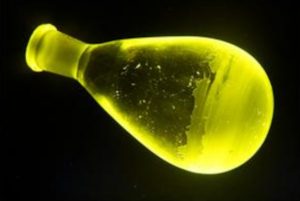
Ultra-fast spectroscopy, x-ray crystallography and theoretical study revealed a molecule with internal coincidences that favour yellow narrow-band triplet emission (phosphorescence) with little energy wasted as singlet emission (fluorescence) or heat.
These internal processes are also largely self-contained, allowing it to function independent of its immediate physical or chemical environment.
“Our research has led to a clearer understanding of the mechanism behind this molecule’s performance than any previous organic phosphorescent material,” said Tani. “Nonetheless, we believe there is still much to explore, and we are excited about its potential applications.”
Detailed descriptions of this unusual molecule are available in the paper ‘Fast, efficient, narrowband room-temperature phosphorescence from metal-free 1,2-diketones: rational design and mechanism‘, published in Chemical Science, and available to read without payment.
 Electronics Weekly Electronics Design & Components Tech News
Electronics Weekly Electronics Design & Components Tech News