The regulations are extensive and complicated but two things need to be appreciated when selecting a power supply for a medical product:
• A power supply that is not ‘medically approved’ but conforms to IEC/EN 60950 can be entirely suitable for some medical applications.
• A power supply that’s ‘approved for medical applications’ will not necessarily enable the end product to gain qualification IEC/EN 60601-1.
Two related factors contribute to this apparent anomaly. First, medical products are classed either as medical devices (MD) or in-vitro diagnostic medical devices (IVD). Second, IEC/EN 60601-1 sets out different requirements for the protection of operators and patients.
MDs are connected to, or used in close proximity to patients. Examples include respirators, syringe pumps and cardiac monitors. IVDs such as blood glucose analysers, centrifuges and other laboratory equipment will not normally come into contact with patients.
It is the responsibility of the medical product manufacturer to determine the likelihood of a patient coming into contact with such devices through a formal risk assessment process (ISO14971). Where there is deemed to be no significant risk of this happening, IEC/EN 60601-1 norms do not apply and a power supply conforming to IEC/EN 60950 can often be used.
Means of Protection (MOP)
Medical devices must incorporate one or more Means of Protection (MOPs) to isolate patients and operators from the risks of electrocution. A MOP can be safety insulation, a protective earth, a defined creepage distance, or an air gap or other protective impedance. These can be used in various combinations.
It’s important to understand that a protective earth is different from a functional earth. The latter is used for EMI suppression. A protective earth is a fail-safe circuit whose only purpose is to protect people from electric shock.
Creepage is the shortest path between two conductive parts (or between a conductive part and the bounding surface of the equipment) measured along the surface of the insulation.
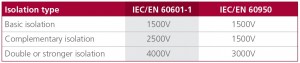
As mentioned earlier, IEC/EN 60601-1 3rd edition differentiates between the risk to patients and the risk to operators. A MOP can therefore be classified as Means of Patient Protection (MOPP) or a Means of Operator Protection (MOOP). The isolation, creepage and insulation requirements for each are set out in Figure 1.
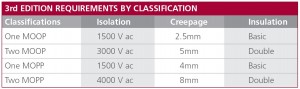 The main difference between one MOOP and one MOPP is primarily one of permissible creepage distance. Both requirements are satisfied using Basic insulation. To achieve 2 x MOPP qualification the isolation test is particularly demanding at 4000 Vac and the creepage distance of 8mm is twice that required for one MOPP.
The main difference between one MOOP and one MOPP is primarily one of permissible creepage distance. Both requirements are satisfied using Basic insulation. To achieve 2 x MOPP qualification the isolation test is particularly demanding at 4000 Vac and the creepage distance of 8mm is twice that required for one MOPP.
Figure 1: IEC/EN 60601-1 3rd edition requires differing levels of isolation, insulation, creepage, and leakage current depending on the MOP level.
The withstand test voltages are higher for under IEC/EN 60601-1 than under IEC/EN 60950, as set out in Figure 2.
Figure 2: Test voltage requirements for medical vs. industrial power supplies.
In any AC-connected power supply there will be leakage current caused by capacitive coupling across power transformers and by the Y-class filter capacitors that are necessary to maintain EMC performance. In IEC/EN 60601-1 the leakage current limits are the same for MDs of all classes: no-indirect patient contact, direct patient contact, and direct patient contact with the heart.
It should be noted that where medical products are to be sold in the North American market, conformance to UL 60601-1 dictates that the maximum allowable leakage current is 0.3mA, rather than 0.5mA.
Where a power supply conforming to IEC/EN 60950 is selected to power an IVD, the leakage current limits of IEC/EN 60601-1 must still be met.
Power supply selection considerations
For IVD applications where a risk assessment demonstrates that there is no significant chance of a patient coming into contact with the product, MOOP requirements may be satisfied using a power supply that simply conforms to IEC/EN 60950. However, it is the responsibility of the medical product manufacturer to demonstrate that the appropriate standard of safety has been achieved.
Power supplies that meet 2 x MOPP standards provide the highest level of protection. But, as noted earlier, when a power supply manufacturer describes a unit as ‘approved for medical applications’ it does not mean that the unit provides two MOPPs.
If an AC-DC power supply only provides one MOPP, an additional MOP (MOOP or MOPP) may be introduced into the final product by designing the power system architecture to include an isolated DC-DC converter. This adds cost and complexity but may be the preferred solution, particularly where multiple DC rails are needed in the medical product.
Historically, medical power supplies were produced in relatively small quantities and were sufficiently different from industrial units that the price premium over the latter was significant. However, in recent years, the growth in volumes of medical equipment and advances in both components and power supply design techniques have reduced the price differential. Many units are now listed as approved for both ITE and medical applications.
It can be advantageous to specify a 2 x MOPP power supply for most applications. Even if there is a small price premium over IEC/EN 60950 units, this approach simplifies qualification of the end product. It may also reduce inventory costs by enabling a common power supply to be used in more than one medical product.
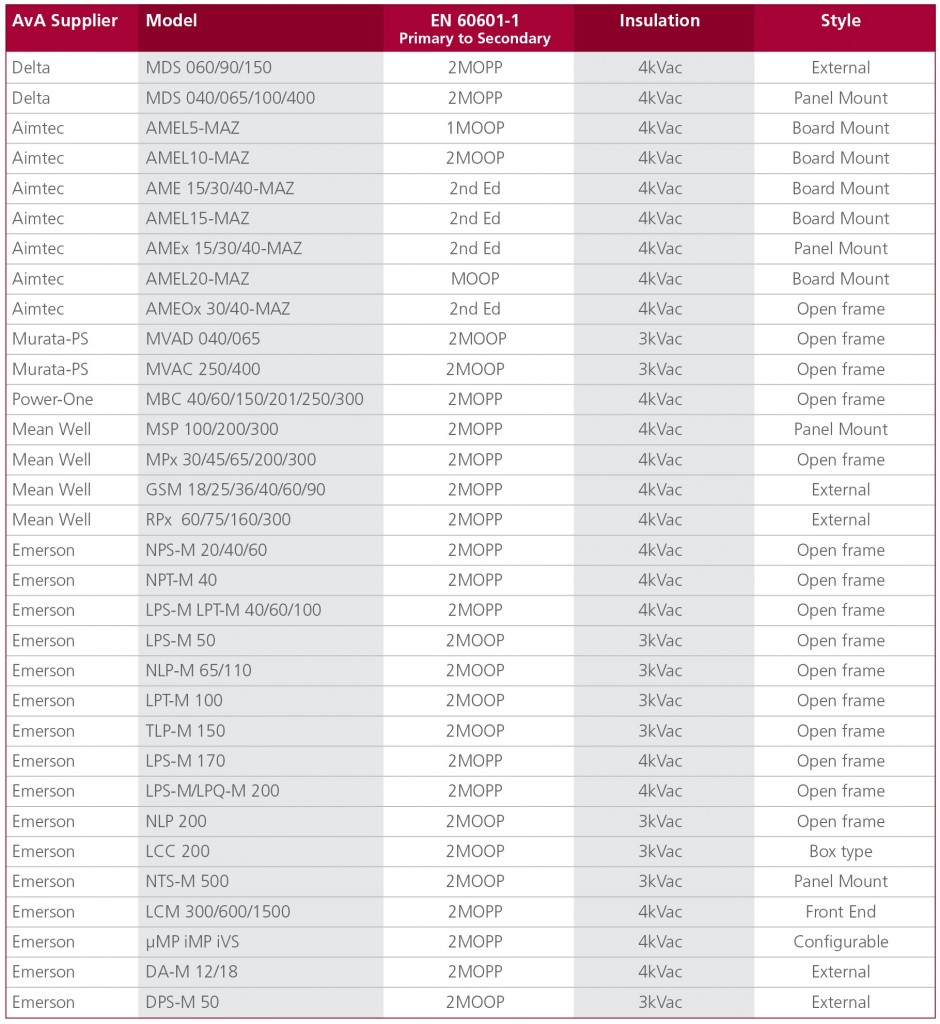 Recently introduced AC-DC power supplies are more likely to meet the 2 x MOPP requirement than older designs. Examples of power supplies from six leading manufacturers, together with their EN/UL 60601-1 approval status, are shown in Figure 3.
Recently introduced AC-DC power supplies are more likely to meet the 2 x MOPP requirement than older designs. Examples of power supplies from six leading manufacturers, together with their EN/UL 60601-1 approval status, are shown in Figure 3.
Figure 3: Delta, Emerson, Power One and Mean Well now all offer medical power supplies with 2 x MOPP qualification
In general, if a manufacturer has gone to the effort of designing a power supply for 2 x MOPP isolation, it will make a feature of this on the data sheet.
Wherever a more general term is used, such as ‘approved to IEC/EN 60601-1’, it is the responsibility of the medical device manufacturer to ascertain the degree protection to which the power supply is certified.
Philip Lechner works for Avnet Abacus
 Electronics Weekly Electronics Design & Components Tech News
Electronics Weekly Electronics Design & Components Tech News