The company claims it will be the first pure-play foundry with 300mm capability.
“With Rogue Valley Microdevices’ investment in  300mm MEMS capability at our new Palm Bay, Florida fab, we’re poised to empower our customers with significant competitive advantages, facilitating their journey from initial concept to the successful commercialisation of their MEMS and sensor designs,” says founder and CEO Jessica Gomez (pictured).
300mm MEMS capability at our new Palm Bay, Florida fab, we’re poised to empower our customers with significant competitive advantages, facilitating their journey from initial concept to the successful commercialisation of their MEMS and sensor designs,” says founder and CEO Jessica Gomez (pictured).
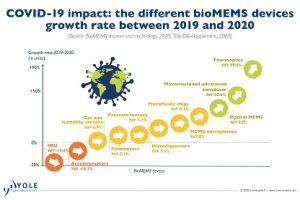
MEMS devices like microneedles are used in the healthcare industry for drug delivery and sensing applications such as continuous glucose monitoring patches for diabetics, along with emerging applications such as vaccine delivery and skin cancer treatment.
When microneedle companies, and other makers of disposable bioMEMS can build their devices on 300mm wafers, they gain the economies of scale presented by larger substrates, resulting in higher margins and potential entry into new markets.
These benefits apply to industries beyond biomedical devices, and include applications in automotive, agricultural, and industrial markets.,
Rogue Valley Microdevices is a full-service precision MEMS foundry that combines state-of-the-art process modules with the engineering expertise to go seamlessly from custom design to manufacturing.
Specializing in MEMS and sensors manufacturing—including microfluidics and lab-on-chip platforms—Rogue Valley Microdevices offers a flexible equipment set and the ability for customers to start with smaller batch sizes, serving a key function in the commercial MEMS manufacturing ecosystem.
The company claims to maintain the broadest and most comprehensive set of wafer services commercially available—with over 50 unique dielectric and conductive thin films and all services performed in its own class 100 cleanroom.
For more information, email: info@roguevalleymicro.com, visit: https://roguevalleymicrodevices.com,
 Electronics Weekly Electronics Design & Components Tech News
Electronics Weekly Electronics Design & Components Tech News